BIO112 Laboratory Guide #9
DIVERSITY OF THE PLANTS
INTRODUCTION
Plants are photosynthetic autotrophs that capture radiant energy from the sun and convert it to chemical energy in the bonds of carbohydrates. All plants are multicellular eukaryotes with chlorophyll a and chlorophyll b as their photosynthetic pigments and cell walls made of cellulose (unlike the chitinous cell walls of fungi). They also store their carbohydrates as starch, which helps differentiate them from some of the algae to which they are related. Plants are most closely related to the green algae, Division Chlorophyta, which also use starch for food storage; indeed, the three groups of algae (Chlorophyta, Rhodophyta, Phaeophyta) were historically sometimes classified not as protists but in Kingdom Plantae. True plants differ from algae in three major ways: 1) all are multicellular with tissues, 2) they are terrestrial (except when they have secondarily evolved an aquatic lifestyle, e.g., water lily), and 3) they are embryophytes, meaning their embryos are enclosed in protective maternal tissue.
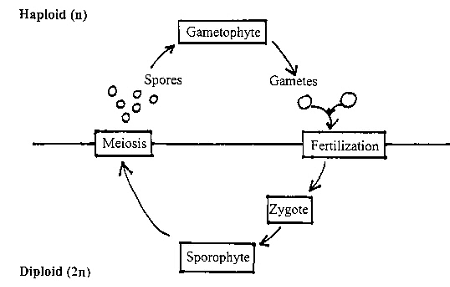
Another feature that unites the Kingdom Plantae is an aspect of their life cycles: all plants exhibit alternation of generations, the alternation between distinct, multicellular, diploid and haploid phases of their life cycle. The diploid phase, called the sporophyte, produces haploid spores by meiosis, which then grow into the gametophyte. The gametophyte then produces gametes (eggs and sperm) by haploid mitosis. Egg and sperm unite in fertilization (syngamy) to form a diploid zygote. The zygote grows through an embryo phase to become the next sporophyte generation. There is thus an alternation between the sporophyte and gametophyte stages. A generalized life cycle for plants is illustrated in the figure below:
In this lab you will learn the details of the life cycles of four representative plants: moss, fern, pine, and lily. All exhibit alternation of generations, but the details differ, as well as the relative sizes and independence of the gametophyte and the sporophyte (the larger phase is said to be “dominant”). Most of the differences in the life cycles of the various plant divisions can be related to the evolution of traits that permit greater terrestriality and independence from water.
The first part of this laboratory exercise gives an overview of the eleven divisions in the kingdom Plantae. These divisions occur in four groups with similar characteristics:
1) Nonvascular plants:
Hepatophyta (liverworts)
Anthocerophyta (hornworts)
Bryophyta (mosses)
2) Ferns and fern allies (seedless vascular plants):
Lycophyta (club mosses, quillworts, spikeworts)
Minoliophyta/Pterophyta (ferns, whisk ferns, horsetails)
3) Gymnosperms (seed plants with “naked seeds”):
Ginkgophyta (ginkgo)
Cycadophyta (cycads)
Gnetophyta (gnetophytes)
Coniferophyta (conifers)
4) Angiosperms (seed plants with seeds enclosed in fruits):
Anthophyta (flowering plants)
In the second part of this lab you will conduct an experiment on water transport in a vascular plant.
After completing this laboratory you should be able to:
1) list the distinguishing characteristics of members of the kingdom Plantae
2) identify and outline the major features of the natural history of the major groups of Plants
3) draw and label the life cycles of four representative plant species (moss, fern, pine, lily) and relate these to the the evolution of terrestriality in plants
4) understand the use of a simple potentiometer to monitor water transport in a vascular plant
PART I. DIVERSITY OF THE PLANTS
Materials
Materials at stations arranged on the lab benches. These consist of fresh materials
and live specimens as well as preserved specimens, prepared microscope slides,
and other written and illustrated materials.
Microscopes, slides and cover slips, forceps, lens paper.
Dissecting stereo microscopes.
Procedure
1. A dichotomous key which outlines the major distinguishing features of each vertebrate class is provided below.
2. Starting at any station, read about the materials in your lab manual and observe the specimens on the lab benches. You may be asked to make a wet mount of a specimen, or examine a prepared slide, or look at an entire specimen, depending on the station.
3. Be able to recognize all specimens as well as their distinguishing characteristics.
4. Make detailed diagrams of the life cycles of the moss, fern, pine, and lily. Use your text, the photographic atlas, the life cycle posters, and live and preserved materials to help you construct the diagrams.
5. Dissect a lily flower and draw a picture of its parts. Learn all the parts of a flower and their reproductive functions.
Study Suggestions
1. Make sketches and detailed notes on specimens. This will help you to observe the specimens more carefully, as well as help you study later.
2. Plan to view the specimens once or twice more before the lab test. Test yourself by attempting to identify the specimens without first looking at their labels.
3. The words in bold print in the extended guide below are words you should know and/or structures you should be able to identify or describe
A Dichotomous Key to the Plant Divisions
All of the following groups are eukaryotic, photosynthetic autotrophs with chlorophyll a and cellulose-rich cell walls. They are all multicellular embryophytes.
1. Nonvascular plants; gametophyte generation dominates over sporophytegenration Mosses and Moss Allies
2. Plants low-growing with stemlike and leaflike appendages; sporophyte
usually a capsule atop a slender stalk BRYOPHYTA
2. Plants low-growing, flat, sheet-like and lobed; sporophyte umbrella-likeor horn-like
3. Lack stomata; sporophytes associated with umbrella-like structures;may have gemmae cups for asexual reproduction HEPATOPHYTA
3. Stomata present; sporophyte an elongated hornlike capsule;gemmae absent ANTHOCEROPHYTA
1. Vascular plants; sporophyte generation dominates over gametophyte
generation
2. Seedless plants; reproduce by spores only Ferns and Fern Allies
3. Plant apparently just a green stem, lacking leaves; if with scalybranches, these arise in whorls from nodes of hollow,
jointed, silica-rich stem
4. Plant with stem and flattened branches only; dichotomouslybranched; simple spore-producing structures (sporangia) at
tips of stems PTEROPHYTA, in part (Psilotum nudum)
4. Plant with silica-rich, round, jointed, hollow stem; may havescaly branches arising in whorls from nodes of stem; "cone"
(strobilus) of sporangia present at tip of stem PTEROPHYTA,
in part (Equisetum sp.)
3. Plant with true leaves and leafy appearance
4. Plant with leafy fronds; low-growing to tree-sized; sporangiaeither in clusters (sori) on underside of leaf or borne entirely on
specialized "fertile" fronds PTEROPHYTA
4. Leaves various (either moss-like or scaly (Lycopodium,Selaginella), or quill-like if an aquatic plant (Isoetes);
all low-growing; sporangia borne in strobilus at stem tip,
or in leaf axils, or at fleshy base of leaves (Isoetes)
LYCOPHYTA
2. Seed plants
3. Flowers absent; seed naked, not enclosed in ovary (or fruit)Gymnosperms
4. Trees (sometimes shrubs); either broad-leaved or with needle-likeor scale-like leaves
5. Seeds usually borne in woody cones; leaves needle-like orscaly; usually evergreen CONIFEROPHYTA
5. Seeds with ill-smelling, fleshy coat; deciduous tree withfan-shaped leaves with parallel veins GINKGOPHYTA
(Ginkgo biloba)
4. Shrubs or trees; leaves palm-like, or in the shrub Ephedra,leaves small and scaly and stem photosynthetic.
5. Shrub with many branches; leaves scale-like and stemsphotosynthetic and jointed (not hollow as in Equisetum);
desert habitats GNETOPHYTA (Ephedra)
5. Shrubs or trees with distinct trunk or with stem mostlyunderground; leaves palm-like; tropical or subtropical
habitats CYCADOPHYTA
3. Flowers present; seed enclosed in ovary, maturing into fruit:Angiosperms ANTHOPHYTA
NON-VASCULAR PLANTS MOSSES AND MOSS ALLIES
There are three divisions of nonvascular plants: Bryophyta (mosses), Hepatophyta (liverworts), and Anthocerophyta (hornworts). Members of this group are nonvascular and lack true leaves and roots. They absorb water and nutrients directly through their surfaces and are therefore restricted to moist habitats. They also require water to complete their life cycles, since the sperm swim from the antheridium to the archegonium. The gametophyte (haploid) generation is dominant over the sporophyte (diploid) generation.
Study the poster of the moss life cycle, which is representative of the life cycles of the bryophytes. Compare this to preserved and live moss samples. Can you identify the gametophyte generation, sporophyte generation, capsule, and operculum?
It would be a good idea to make your own detailed diagram of the moss life cycle, labeling all key stages and structures.
Compare the moss life cycle to the preserved mount and live specimen of the Marchantia (liverwort). Can you find the thallus, gemmae cups, antheridiophores, and archegoniophores?
Examine the live specimen of a hornwort. To what part of the life cycle do the horns corespond?
VASCULAR SEEDLESS PLANTS - FERNS AND FERN ALLIES
The two divisions that make up the ferns and their allies are seedless vascular plants with the sporophyte generation dominating over the gametophyte generation. As with the bryophytes, the sperm are free-swimming, meaning that water must be present at some point in the life cycle of these plants for them to prosper in a given habitat. Lacking seeds, they propagate via air-borne spores. View living specimens and various preserved plants.
Division Lycophyta: club mosses, quillworts, and spikeworts
Lycopodium, Selaginella, Isoetes are the three genera. Shown here is a real fossil of Lepidodendron, a tree-sized lycopod. These species made up the “coal forests” during the age of dinosaurs. The Carboniferous was their heyday.
Examine the preserved and live lycophyte specimens.
Division Monilophyta/Pterophyta: ferns
See the prepared slide of he fern prothallium (gametophyte generation) with a young fern embryo (sporophyte generation growing out of it. Study the poster of the fern life cycle, which is representative of the life cycles of the seedless vascular plants.
Division Pterophyta also includes the horsetails and scouring rushes, which are distinctive enough that they sometimes classified in their own division (Sphenophyta). Equisetum is the only genus. These plants are high in silica, so their stems are not good for the teeth of herbivores.
The whisk ferns were likewise formerly in their own division (Psilophyta), but are now considered to be true ferns. Only one species (Psilotum nudum) occurs in the U.S.; it can be found along the Georgia coast. Characteristics include dichotomous branching, and lack of true leaves or roots.
It would be a good idea to make your own detailed diagram of the fern life cycle, labeling all key stages and structures.
GYMNOSPERMS
The uniting feature of this group is the presence of a seed, but with no ovary enclosing the seed. Thus the name gymnosperm, which means “naked seed”. Because there is no ovary, technically these plants have no true fruits, though some (e.g., ginkgo, red cedar) have fleshy coatings on the seeds.
Ginkgophyta: The ginkgo.
A very ancient lineage, with just one remaining species: Ginkgo biloba, a common ornamental tree species. Female trees are not widely planted because their seeds have a foul-smelling fleshy coat, but there is a female ginkgo on campus, to the northwest of Tate Hall.
Cycadophyta: The cycads.
One species (Zamia pumila) is native to the U.S.; it occurs in Florida and southern Georgia. See the living specimen as well as cycad cones and seeds on display.
Gnetophyta
Ephedra is the only species native to the U.S. It is found out west in arid habitats.
Coniferophyta: The Conifers.
Cone-bearing, needle-leafed trees and shrubs. Lots of species native to Georgia, including bald cypress (Taxodium), red cedar (Juniperus) and pines (Pinus), altogether representing three families. Study the poster of the pine life cycle and the preserved and live materials that accompany it.
It would be a good idea to make your own detailed diagram of the pine life cycle, labeling all key stages and structures.
ANGIOSPERMS
Angiosperm means “vessel seed” which refers to the protective ovary enclosing the seed. The ovary develops into the fruit. These plants are called the flowering plants because they are the only group that possesses true flowers. This is the most species rich group of all plants, with almost 90% of all species. This group arose about 130 million years ago, according to the fossil record.
Anthophyta. Flowering Plants
This division has two classes: Monocotyledones (monocots) and Dicotyledones (dicots). The monocots include grasses, lilies, orchids -- they are primarily herbaceous (exceptions being the palms and bamboos).
What are the differences between dicots and monocots? Consult your text and photographic lab atlas to discover how these two classes differ in terms of leaf venation, number of flower parts, the arrangement of vascular bundles, and number of cotyledons (seed leaves).
Study the flowering plant life cycle as exemplified by the Lily (Lilium). See poster.
Also see slides of Lilium anthers and pollen tetrads, 8-nucleate embryo sac (= “mature female gametophyte”), stigma and pollen tubes. Be sure you understand the concept of double-fertilization.
It would be a good idea to make your own detailed diagram of the lily life cycle, labeling all key stages and structures.
PART II. PLANT PHYSIOLOGY: MEASURING TRANSPIRATION.
In this laboratory exercise you will measure the rate at which water moves out of a plant in the process called transpiration. Transpiration is the evaporative loss of water from leaf surfaces. Water loss via transpiration can be considerable; plants transpire about 90% of the water they absorb. The study of water loss via the xylem is very amenable to experimental manipulation and is better understood, both anatomically and functionally, than movement of materials in the phloem. You will design your own experiment to simulate rates of water loss from plants under one or more of the following conditions: hot sunny days, windy days, humid days, or defoliation by herbivores.
The device you will use to measure transpiration is called a potometer (poto = drink). This simple device is constructed from a glass bottle, a rubber stopper, and a pipette (see illustration). During assembly, you must be careful not to allow any air to enter the cut plant stem. All seals must likewise be very snug and secure to prevent water leakage.Materials
Potometers (glass bottle, a rubber stopper (2 holes), one ml pipette)
Petroleum jelly and toothpicks
Cut branches from plants, e.g., geranium, Coleus, or a woody plant
Parafilm
Timers/stop watches
Razor blades, scalpels, small pruning shears
Large syringes (without needle tips) for purging air bubbles
Plastic bins for submerging plants while cutting stems and assembling potometers
Electric fans, lamps, plastic bags for experimental treatments.
Experimental Procedure
1. Wash the potometer with soap and water and rinse in DI water. Scrupulously clean glassware is essential to avoid formation of bubbles on the inside of the potometer, which will lead to erroneous results by making the water level rise in the pipet.
2. Use a cut branch from a plant such as a geranium or some small woody plant and hold the stem under water while cutting about 1-2 cm from the end. This will remove any dead tissue and air bubbles that may have entered the xylem. Cut on an angle to increase the surface area of the cut stem. The plant must remain immersed in water after cutting.
3. Wrap the stem with several tapered layers of parafilm where the stem will pass through the rubber stopper -- the parafilm will help to stop leaks. Carefully insert the cut stem into the rubber stopper; the stem must fit snugly inside the tubing, but should not be crushed.
4. Fill the bottle to the brim with DI water (work slowly and carefully to avoid injecting gases into the water), then insert the rubber stopper (with pipette and plant stem) into the top of the bottle. (Note that there will be some spillage of water when the stopper is inserted, so be sure to do this step in the sink or over a plastic basin. (This step ensures that any air bubbles are purged from the bottle. If any leaks or bubbles are detected the process must be started over, ensuring especially that the cut stem is inserted snugly in the tubing.) Place the potometer on a tray, then dry off the apparatus to enable you to detect any leaks that might develop. Finally, seal around the base of the stem with petroleum jelly to eliminate leaks. The plant needs to be left alone for 10 minutes to equilibrate to room conditions before any readings are made.
5. As water leaves the plant by transpiration it is replaced by water from the jar. The rate of water loss can be determined by measuring how far the meniscus moves in the pipette over a specified period of time. Read the meniscus at 3-minute intervals for at least 30 minutes; you should have at least 10 readings. Record data on the data sheet provided. The normal rate of water loss for the plant represents the transpiration rate under controlled conditions.
6. Now determine the rate of transpiration under experimental conditions of your own design. Your task is to develop a hypothesis about how changes in air movement, light, temperature, humidity, herbivory, and the opening and closing of stomata may influence transpiration, and then design an experiment to test your hypothesis. Your experiment should be reasonable in the sense that it mimics the conditions plants might actually experience in nature. Again, you will need at least 10 readings taken over 30 minutes for your experimental treatment. Each group in the lab section will perform a different experimental manipulation.
7. After lab, you will be given a data sheet summarizing the results of all the groups in your lab section. This data sheet should go in your lab manual.
8. In your lab notebook, include the following:
- a clear statement of your hypothesis.
- a description of the methods used in your experimental treatment. Be sure to give enough information about the setup and manipulation that someone else could replicate your experiment if desired.
Data Analysis and Questions
A. Graphically Representing Your Results:
- For each of the experimental treatments done by the student groups in your lab section, you will produce a line graph of water loss (ml/minute) over time. The "time" axis will be 60 minutes long, the first 30 minutes representing the control conditions and the next 30 minutes representing the experimental conditions.
- For each of the experimental treatments done by the student groups in your lab section, you will now produce a bar graph of average water loss (ml/minute). Each figure will have two bars: one for the control and one for the experimental manipulation.
- All figures should be numbered; furthermore, they require explanatory legends so that the figures stand alone and could be interpreted by someone unfamiliar with your work.
B. Questions:
- Was your hypothesis supported by the data?
- Explain the physiological basis for differences (or lack thereof) in the transpiration rates under controlled conditions versus the experimental conditions.
- If you wanted to compare plant transpiration rates among different student groups, how might you need to manipulate the data to make it comparable? (Hint: the plants probably differed in size.
Plant Physiology Practicum:
Transpiration Data Sheet
Date: ____________ Group Members: __________________________
Plant Species: ________________________
Type of experimental manipulation:
(e.g., light, fan, bagging, leaf removal, heat, etc.)
Control Treatment started at: __0__ minutes, _____ o’clock, pm
Control Treatment terminated at: _____ minutes, _____ o’clock, pm
Experimental Treatment started at _____ minutes, _____ o’clock, pm
Experiment terminated at: _____ minutes, _____ o’clock, pm
Time Potometer Reading (ml)
Control: (minutes) e.g., 0.815 ml
0
3
6
9
12
15
18
21
24
27
30
Time Potometer Reading (ml)
Experimental: (minutes) e.g., 0.815 ml
30
33
36
39
42
45
48
51
54
57
60